![]()
 When adding soluble powder or liquid in the vortex, there will be rapid downward transport of the contents. Mixer velocity is the most important parameter, and this will vary from application to application in accordance with the process requirements. |
 When the additive powder or liquid meets the top of the impeller the axial movement will be transformed into a radial acceleration from the center of the impeller towards the wall of the vessel. |
 During steps 1 and 2 the additive will be transported effeciently and mixed into the bulk liquid. In addition, the pumping effect along the wall of the vessel will ensure homogeneous mixing, avoiding any localized concentration gradients. |
 |
||
|
A unique magnetic mixer The Sterimixer® from Roplan is probably the most used magnetic mixer for pharmaceutical applications worldwide. The mixer consists of the following major components: impeller, bearing, weld plate, drive unit and control box. All wetted parts of the impeller and the weld plate are made of polished EN No. 1.4404 (AISI 316L) stainless steel. Two impeller designs are available: Aseptic design SMA Impeller aseptic type SMA, is ideal for critical applications. The SMA impeller has horizontal flow-channels which, using the differential pressure created during rotation, ensure continuous purging of liquid through the bearing and from beneath the impeller. This patented feature optimises the in situ cleaning and steam-sterilization of the impeller assembly, and has been proven in a wide range of applications. Open design SMO Impeller open type SMO, for less critical applications, is a cost effective alternative; both impeller types use the same bearing and weld plate assembly and are fully interchangeable. The SMO is the natural choice for applications where shear forces are not critical. The SMO impeller shows excellent CIP performance and can be cleaned efficiently using typical in-vessel equipment, purging directly to drain. |
A wide choice of models Sterimixer® models are available for vessel volumes ranging from 5 to 30 000 liters, with or without control cabinets and with various types of drive units: AC, direct coupled frequency drive "Motec", DC and air, together with Explosion Proof options. The Sterimixer® is manufactured in accordance with relevant industry standards and requirements such as: applicable pressure vessel codes, material traceability requirements and the EU Machine Directive 89/392/EEC, as well as GMP. Total quality concept The manufacturing of Sterimixer® is quality assured and certified to be in accordance with ISO 9001 by Lloyds Register Quality Assurance and in addition, benefits from our extensive experience within the pharmaceutical industry. References Worldwide references for the Sterimixer® include the manufacture of blood protein fractions, delicate cell suspensions, insulin, LVPs, buffer solutions and oral liquids. Using this background together with a history of manufacturing excellence and full technical support, Roplan can guide you to obtain the most user-friendly and cost-effective solution for your application. The Roplan Development Centre and demonstration equipment are available for confidential customer product testing and CIP tests. We offer facilities for process optimization under controlled technical and financial conditions. |
||||
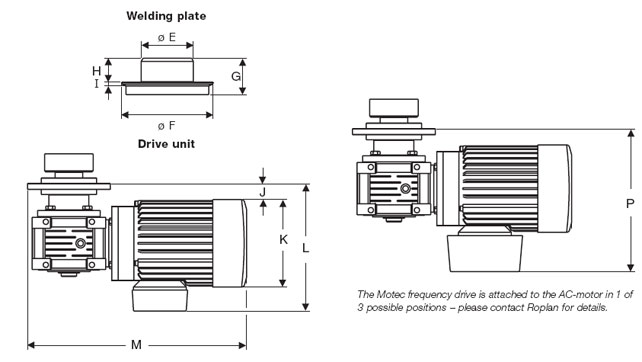
| Principal Drawing | |||||
   |
|||||